





















EUR
en
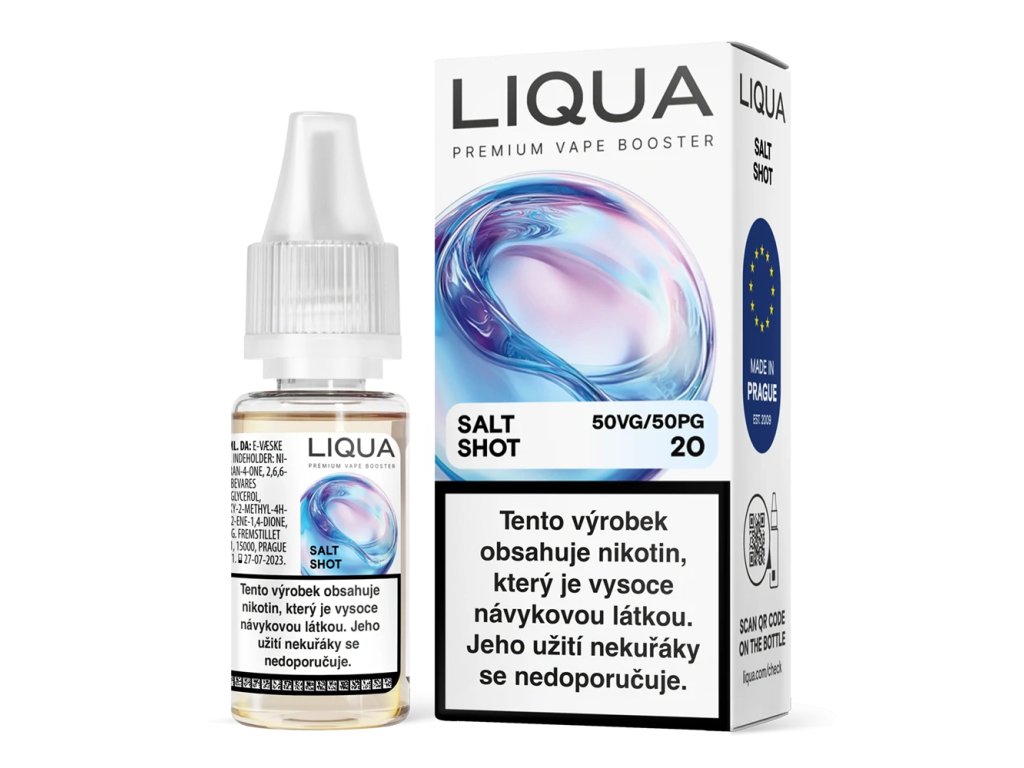
This product contains nicotine. Nicotine is an addictive chemical.
What is PMTA? PMTA (Premarket Tobacco Application) is an application required by the FDA to be filed for the legal listing of any new tobacco products after February 15, 2007. The FDA can approve or deny a product based on its risks and benefits to the general public.
Geek Bar is responsible for our customers, supporters and partners. No matter how the market is changing, we are fully committed to meeting all of our legal and regulatory obligations. In order to facilitate vaping industry become well-regulated and well-developed to the next level. We have been working on Premarket Tobacco Product Applications for quite some time and have submitted PMTAs to the FDA for our products, including no flavored products aside from tobacco and menthol pods. We are confident in the content and quality of the materials we submitted with our application.
Bookmark
Daniel Féau processes personal data in order to optimise communication with our sales leads, our future clients and our established clients.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.